Summary:
- Paxlovid treatment consists, each dose, of three pills: two of nirmatrelvir and one of ritonavir.
- Ibuzatrelvir fits right into the active site of the coronavirus main protease.
- So as long as we have the current coronvirus with us – and it looks to be settling in for a good long while – this looks like it’ll be a front-line therapy once it completes testing and is approved by the FDA.
Monty Rakusen
Here’s a look at the next iteration of Pfizer’s (NYSE:PFE, NEOE:PFE:CA) now-well-known antiviral, Paxlovid. As those who have taken it know, the treatment consists, each dose, of three pills: two of nirmatrelvir (the antiviral itself) and one of ritonavir (updated to fix those numbers, since I’d clearly blocked out the experience of taking it!). And even though that has a generic name that tells you of its antiviral origins, ritonavir has no antiviral activity towards coronaviruses at all. It’s there as a very effective inhibitor of CYP3A4, one of the most common drug-metabolizing enzymes in the liver, which would otherwise tear down the nirmatrelvir to therapeutically useless levels.
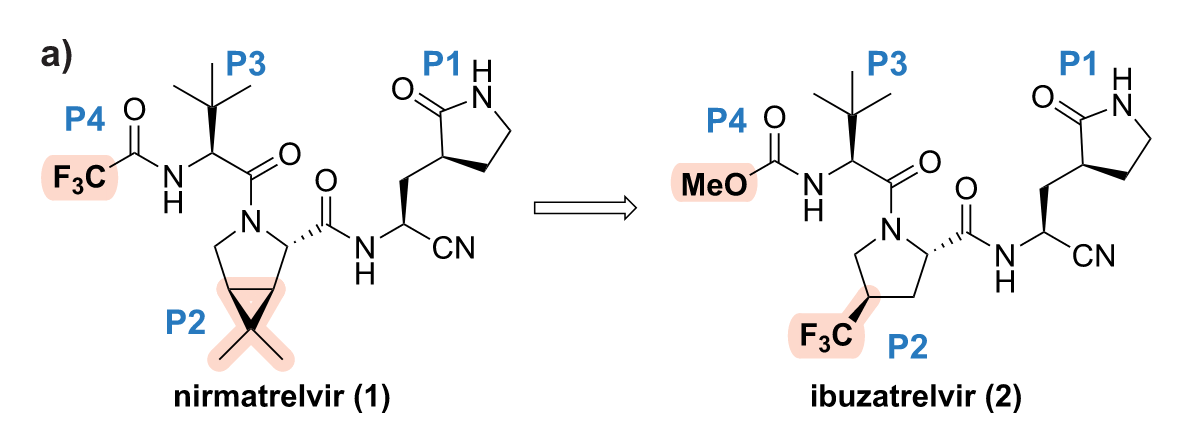
That’s not the optimal way to do things, clearly, but time was tight! Taking one pill would be better than three in general, and it would be especially useful to people who are on various other medications not to take a CYP3A4 inhibitor (which could easily cause them to be exposed to far more of their usual prescription drugs than they would experience normally). As the article linked above shows, Pfizer’s folks have been working on this problem, and the result is ibuzatrelvir, shown above. It’s a very similar molecule in many ways, and fits right into the active site of the coronavirus main protease. In both cases, it’s the nitrile that reacts with the active-site Cys residue (Cys145) to inhibit the enzyme. Here’s a J. Med. Chem. article from earlier this year with more details.
But replacing that dimethylcyclopropyl-fused proline with a trifluoromethylproline (the P2 region) makes the amide coming off the ring nitrogen more rotatable (an effect you can see clearly in variable-temperature NMR spectra), and this seems to be a real factor in improving the bioavailability of the compound after oral dosing. Not being quite so married to a single conformation, the crystalline form of the new compound dissolves much more easily. Meanwhile, switching to a methyl carbamate out in the P4 region gives you even more potency against the viral enzyme while substantially decreasing the compound’s clearance from the blood.
Ibuzatrelvir looked clean through the other usual tests (no CYP activities of its own, no red flags on selectivity versus other enzymes whose active-site residues are inhibited by nitrile warheads), and it showed very strong potency in animal models. Phase II results were reported from human trials earlier this year, and its activity looks just as good as you would have expected, with no co-dosing of ritonavir needed. And what’s more, there were no reports of bitter or metallic tastes during the trial. This one will be taken on the same schedule as Paxlovid (twice a day for five days), but it looks like it will be substantially easier to dose.
So as long as we have the current coronvirus with us – and it looks to be settling in for a good long while – this looks like it’ll be a front-line therapy once it completes testing and is approved by the FDA. No one will miss the multiple-pill regimen much, and no one at all will miss that distinctive, annoying taste. No doubt, anything that reminds us of it in coming years will induce weird feelings of early 2020s nostalgia (what a thought. . .)
Disclosure: None.
Editor’s Note: The summary bullets for this article were chosen by Seeking Alpha editors.
