Summary:
- Merck’s recent decline is due to reduced profit guidance and FDA decisions, but shares are at a support level that is likely to hold.
- Keytruda remains a key revenue driver, with potential for continued growth and extended patent protection through a subcutaneous formulation.
- Strategic acquisitions are broadening Merck’s pipeline and should reduce its reliance on Keytruda, despite the short-term profit impacts.
- Merck’s dividend is well-covered, and shares are positioned for appreciation, making current pricing an attractive entry point.
JasonDoiy
After several years of outperformance, Merck (NYSE:MRK) has been declining since the start of the summer, and especially following the company providing a guidance cut to its full-year profit outlook. In the last few weeks, Merck has continued to decline, including after the FDA voting against broadened use of Keytruda in certain patients with stomach cancer. Now, shares have reached the bottom of their recent trading range, and appear to be at a level of support that is reasonably likely to hold. As such, shares offer a reasonable value at risk at current pricing, and a strong likelihood of appreciating in the coming months and quarters.
Last month, the FDA’s Oncologic Drugs Advisory Committee voted 10-2, with one member abstaining, that Merck’s Keytruda and Bristol Myers Squibb’s (BMY) Opdivo are not beneficial for stomach cancer patients who test negative for the PD-L1 cancer biomarker. This decision also compels a change in the labeling information for these drugs, which are indicated as first-line treatments for HER2-negative unresectable or metastatic gastric or gastroesophageal junction adenocarcinoma (“GEJ”).
This label change and failure to obtain a broadening of use reduces the projected future revenue of Keytruda, and this has likely weighed on Merck’s valuation. Also, the reduced profit guidance Merck provided in July is probably still contributing to it recently declining. That reduced profit guidance was due to multiple factors, such as the slowing growth seen by versions of its Human Papillomavirus vaccine, Gardasil, as well as the almost halving of revenue from Merck’s COVID-19 therapy, Lagevrio.
The key driver behind Merck’s reduced full-year 2024 profit guidance was the one-time impact from recent buyout deals. Merck anticipated the cost of those deals will reduce full-year earnings by approximately $0.77 per share.
Merck also recently completed the acquisition of CN201.
Merck, through a subsidiary, has agreed to acquire CN201, a novel investigational clinical-stage bispecific antibody for the treatment of B-cell associated diseases.
“We continue to identify opportunities to expand and diversify our pipeline,” said Dr. Dean Y. Li, president, Merck Research Laboratories. “Early clinical data have provided robust evidence for the potential of CN201 to target and deplete circulating and tissue B cells with the potential to treat a range of malignant and autoimmune diseases.”
Under the terms of the agreement, Merck through a subsidiary will acquire full global rights to CN201 for an upfront payment of $700 million in cash. Curon is also eligible to receive up to $600 million in milestone payments associated with the development and regulatory approval of CN201.
Since the acquisition was agreed to in early August, the charge was not included in Merck’s full-year financial outlook guidance provided on July 30.
Adding this acquisition to the July information means the total cost of recent deals should reduce Merck’s full-year prior guidance by approximately $1.05 per share. The July 30 update included Merck’s acquisitions of EyeBio, and Elanco Animal Health’s Aqua business, both of which Merck completed in early July. Merck indicated it would record a charge of approximately $1.3 billion, or approximately $0.50 per share, for EyeBio, and in the third quarter of 2024, which would have been included in the July 30 update. Merck also completed the acquisition of Harpoon Therapeutics In March for approximately $650 million, or approximately $0.26 per share, but this charge was disclosed in Merck’s initial full-year 2024 financial outlook that it issued in February.
Merck appears to be getting punished for its multiple acquisitions, as well as the decision to pay for them up front. Nonetheless, Merck’s actions were not particularly bad, and actually are what a company like Merck should be doing. The company is still incredibly profitable, but has become highly dependent upon the performance of Keytruda, so Merck is using some of its profits to acquire a pipeline of drugs, as well as existing revenue streams. Elanco’s Aqua business brought with it products across both warm-water and cold-water species, and generated for Elanco an estimated $175 million in revenue and approximately $92 million in adjusted EBITDA, excluding the allocation of corporate costs, in 2023. These appear to be reasonable acquisitions, where Merck needs to broaden its drug portfolio in order to reduce concentration risk related to Keytruda.
Other recent concerns that may be affecting Merck likely include a significant cut to Medicare pricing for Januvia, Merck’s diabetes drug. Among the ten drugs for which the U.S. negotiated lower pricing, Januvia took the largest percentage reduction, with a 79% price cut from the 2023 list price.
The key product within Merck’s portfolio of drugs continues to be Keytruda, which brought in approximately $7.3 billion in sales during the second quarter of 2024, and which is still growing. Keytruda Q2 2024 revenue grew by about 16% year-over-year and beat expectations by approximately $100 million. Last quarter, Keytruda’s revenue made up about 45% of Merck’s total quarterly revenue, which came in at about $16.1 billion. This total revenue figure also represented year-over-year revenue growth of about seven percent.
Another positive result is that should kick in on upcoming earnings reports is from Winrevair, following its FDA approval for pulmonary arterial hypertension this March. Winrevair was acquired along with Merck’s 2021 buying of Acceleron Pharma. Q2 was the first full quarter for Winrevair and the drug brought in about $70 million in revenue, which is likely to increase in coming quarters. Winrevair’s growth in the second half of 2024 may not provide total clarity into the drugs trajectory, as it is possible that the Q2 numbers included some initial stocking of inventory. Of course, that could also be in effect in Q3. In any event, Winrevair appears likely to become a more meaningful contributor to sales over time.
While I anticipate that Keytruda growth will eventually peak due to the significant size it has already reached, as well as its eventual patent expiration, the drug is often approved for additional indications and earlier lines of therapy. It is still entirely possible that Keytruda might sustain double-digit revenue growth for the next few years. Further, Merck is working on a subcutaneous formulation of the drug that could be applicable for about half of the current Keytruda indications. The subcutaneous formulation is currently in Phase 3 development. By coming out with a subcutaneous formulation, Merck could extend patent protection into the late 2030s.
Merck’s chart also indicated that shares are trading at the bottom of its apparent trading range. If this is the case, shares should find a reasonable level of support here, and could be at or close to their near term bottom.
Merck daily candlestick chart (Finviz.com with red lines by Zvi Bar)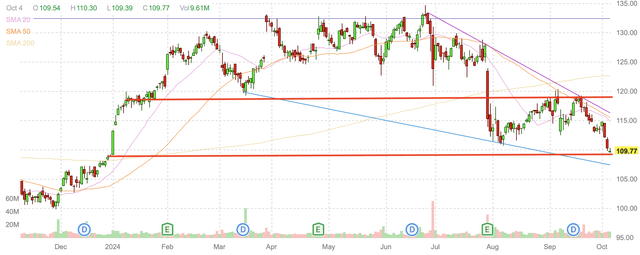
Merck’s performance at the end of 2023 was also reasonably weak, but the company gapped up at the start of the year, when it quickly returned to that level, after which it continued to appreciate towards new all-time highs. This recent decline has taken shares back to a level that acted as both support and resistance in 2023. It also takes shares back to a level that appears in line with the bottom of Merck’s still continuing long-term uptrend over the last three years.
Merck weekly candlestick chart (Finviz.com with red lines by Zvi Bar)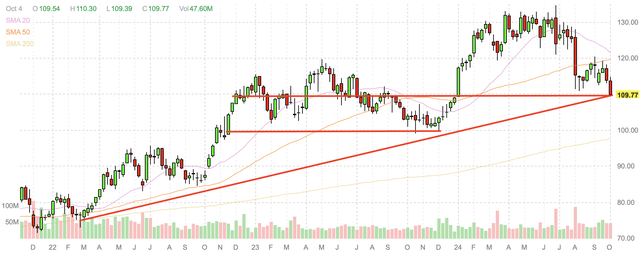
For these reasons, I believe that Merck’s gap down has taken shares to a level that is likely to be supportive, and where it makes sense to acquire shares upon any continued weakness. A break of this level is likely to also find strong support at about $100, but I believe that a drop to that level is unlikely due to the strength of Merck’s revenue on profitability, as well as its dividend.
Merck’s dividend payout is currently an annualized $3.08 per share, or a yield of about 2.8 percent, and it is well covered with Merck’s earnings per share being more than double this level. Also, Merck has raised its dividend in each of the last 13 years, and usually in the fourth quarter of the year, with its 5-year growth rate being just under eight percent. This indicates a reasonable likelihood that a dividend increase is forthcoming later this year.
Risk
Risks to Merck include its continued dependence upon Keytruda. The drug is one of the most successful drugs on the market, and contributed nearly half of Merck’s total revenue. Competing medications should eventually be approved. Also, Medicare may negotiate better pricing, which would reduce Merck’s revenue and profit margins. Keytruda also faces patent expiration in 2028, and this is likely to cause margin pressure. Though Merck is working on a subcutaneous formulation that could extend that patent protection by another decade, it would only apply to about half of Merck’s existing Keytruda prescriptions.
Merck also consistently makes acquisitions, and probably needs to continue to do so in order to reduce its dependence upon Keytruda. It is possible that future acquisitions will further reduce Merck’s profit guidance, and the failure of the pipeline drugs it acquires would also be a negative.
Other concerns include potential poor results from clinical trials. For example, in September, Merck announced that a Phase 3 trial designed to test Keytruda as part of a combination regimen in patients with a type of colorectal cancer did not reach the primary endpoint. Merck also recently discontinued two Phase 3 Keytruda trials due to poor benefit/risk profiles. One, KEYNOTE-867, was evaluating Keytruda plus stereotactic radiotherapy in patients with stage I or II non-small cell lung cancer, and the other, KEYNOTE-630, was evaluating Keytruda for the adjuvant treatment of patients with high-risk locally advanced cutaneous squamous cell carcinoma following surgery and radiation. Merck also stopped another Keytruda late-stage for its use as a first-line treatment of patients with extensive-stage small cell lung cancer.
It is also often the case that pharmaceutical companies and related healthcare equities become volatile and sustain temporary declines prior to presidential elections. This is due to heightened uncertainty around the potential for increased regulatory pressure and potential Medicare price negotiations.
Conclusion
Merck has declined over the last several months due to some reduced profit guidance that is largely due to a series of acquisitions the company made in 2024. Merck also had some poor clinical trials related to Keytruda, and a recent FDA vote that failed to further expand the drug’s usage in certain stomach cancers. Merck now appears to be at a price that acted as both support and resistance in 2023, and which is likely to act as support here. Merck’s revenue continues to appear strong, and Merck is also likely to increase its dividend later this year. For these reasons, I believe Merck shares currently offer a reasonable value at risk and are likely to appreciate over the coming months and into 2025.
Analyst’s Disclosure: I/we have a beneficial long position in the shares of MRK, BMY either through stock ownership, options, or other derivatives. I wrote this article myself, and it expresses my own opinions. I am not receiving compensation for it (other than from Seeking Alpha). I have no business relationship with any company whose stock is mentioned in this article.
Seeking Alpha’s Disclosure: Past performance is no guarantee of future results. No recommendation or advice is being given as to whether any investment is suitable for a particular investor. Any views or opinions expressed above may not reflect those of Seeking Alpha as a whole. Seeking Alpha is not a licensed securities dealer, broker or US investment adviser or investment bank. Our analysts are third party authors that include both professional investors and individual investors who may not be licensed or certified by any institute or regulatory body.
