Summary:
- Affimed has decided to focus on testing AFM13 in combination with Artiva’s AB-101 NK cell product for the treatment of peripheral T-cell lymphoma after underwhelming monotherapy data.
- Peripheral T-cell lymphomas are highly aggressive and difficult-to-treat forms of lymphoma with limited therapeutic options.
- The combination of AFM13 and AB-101 NK cells has the potential to provide a more durable and effective response compared to monotherapy with AFM13.
- However, there are potential limitations and risks associated with the combination therapy and the bar for PTCL is high with the advent of brentuximab vedotin.
- Affimed’s long-shot combination therapy for PTCL is very unlikely to bear fruit. Affimed is a “Sell”.
selvanegra/iStock via Getty Images
Introduction
Affimed (NASDAQ:AFMD), a clinical-stage immuno-oncology company, recently announced that it will no longer pursue monotherapy in peripheral T-cell lymphoma (PTCL) and instead will focus on testing AFM13 in combination with Artiva’s AB-101 natural killer (NK) cell product. This decision was made following the results of Affimed’s phase 2 REDIRECT study, which investigated AFM13 monotherapy in patients with advanced-stage refractory and relapsed PTCL.
In this article, we will examine the reasons behind Affimed’s shift from monotherapy to combination therapy in peripheral T-cell lymphoma. Furthermore, we will evaluate the company’s potential in the wake of investors’ unfavorable reaction to this strategic shift.
Financials
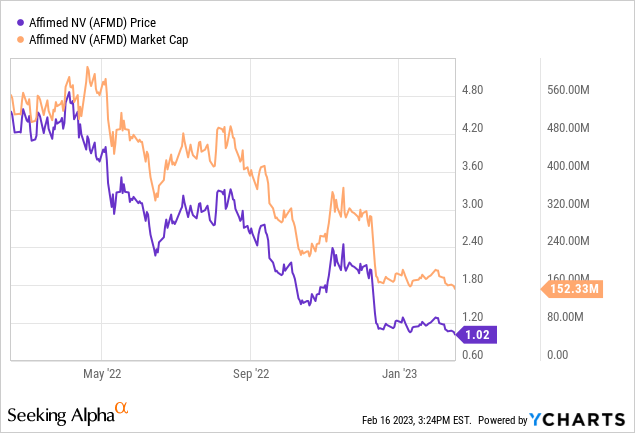
Let’s first review the company’s latest financials. As of September 30, 2022, Affimed had $260.7 million in cash and cash equivalents, which is expected to support operations until mid-2024. The quarter ended September 30, 2022, saw a total revenue of $17.6 million, primarily from collaborations with Genentech and Roivant. Research and development expenses increased by 27% to $29.1 million, while general and administrative expenses increased by 19% to $9.1 million. Net finance income increased by 84% to $3.1 million due to currency fluctuations. The net loss for the quarter was $19.2 million, or $0.13 loss per common share. Affimed is currently valued below its cash and cash equivalents, displaying the disappointment and low expectations from the market.
Affimed Reports Topline Data from Phase 2 REDIRECT Study in Peripheral T-Cell Lymphoma, Decides to Focus on Combination Therapy with AFM13 and NK Cells.
Affimed’s phase 2 REDIRECT study’s primary efficacy measures included an objective response rate (ORR) of 32.4% and a complete response (CR) rate of 10.2%. Secondary and exploratory outcome measures included safety, durability of response, progression-free survival (PFS), and overall survival (OS). The safety profile of AFM13 was consistent with previously reported data from prior and ongoing clinical studies. The median duration of response (DOR) was 2.3 months, the median PFS was 3.5 months, and the median OS was 13.8 months.
According to Dr. Adi Hoess, CEO of Affimed, these data are “remarkable” and confirm that the activation of innate immunity can lead to robust clinical activity. However, the company believes that the combination of AFM13 with NK cells has a higher probability of delivering increased anti-tumor activity and a more durable clinical benefit to address the unmet need in this patient population. Consequently, Affimed will focus its investment on clinical development in the combination of AFM13 with AB-101 NK cells.
Peripheral T-Cell Lymphomas: A Highly Aggressive and Difficult-to-Treat Form of Lymphoma with Limited Therapeutic Options
Peripheral T-cell lymphomas are highly aggressive and one of the most difficult to treat forms of lymphoma with poor prognosis for patients. They account for approximately 10-15% of all non-Hodgkin lymphomas and are often refractory to chemotherapy.
The pathophysiology of PTCL is not well understood, but it is known to be a heterogeneous disease with various genetic and epigenetic alterations. PTCL is characterized by the overexpression of the CD30 antigen, a member of the tumor necrosis factor receptor superfamily, which is also expressed in Hodgkin lymphoma. This makes CD30 an attractive target for therapy in PTCL.
Currently, there are several treatment options available for PTCL, including chemotherapy, radiation therapy, stem cell transplantation, and targeted therapies. However, the efficacy of these treatments is limited, and some patients relapse or develop refractory disease.
The Potential of Combining AFM13 and AB-101 NK Cells in the Treatment of PTCL: Benefits, Limitations, and Risks
AFM13 is a bispecific antibody that targets CD30 on tumor cells and CD16A on NK cells. This allows for the selective activation of NK cells, leading to the destruction of CD30-positive tumor cells. AFM13 has shown promising activity in PTCL, both as a monotherapy and in combination with allogeneic NK cells. The combination of AFM13 with AB-101 NK cells is expected to improve the durability of response and build on the already meaningful activity seen in the REDIRECT study.
The combination of AFM13 and Artiva’s AB-101 NK cells has the potential to provide a synergistic effect in the treatment of PTCL. This is because AFM13 targets the CD30 antigen on lymphoma cells and activates the innate immune system to attack them. The AB-101 NK cells are natural killer cells that can recognize and destroy cancer cells through direct cytotoxicity and the release of cytokines.
Furthermore, the combination of AFM13 and AB-101 NK cells has the potential to provide a more durable response compared to monotherapy with AFM13. This is because NK cells have a longer half-life (as long as a week) compared to monoclonal antibodies (19 hours for AFM13) and can, thus, persist in the body for a longer period of time, providing ongoing immune surveillance against cancer cells.
While the combination of AFM13 and AB-101 NK cells seems promising, there are potential limitations and risks associated with this approach. One major limitation is the potential for toxicity or adverse events. As with any combination therapy, there is always a risk of increased toxicity, which could limit the overall effectiveness of the treatment. In particular, the use of NK cells could lead to cytokine release syndrome, a potentially dangerous inflammatory response that can be fatal in severe cases. This risk will need to be carefully monitored during clinical trials.
There is also the risk that the combination therapy may not be as effective as anticipated. While the preclinical and early clinical data for AFM13 and AB-101 NK cells looks promising, it is still early days for this approach. The combination therapy may not work as well in the larger, more diverse patient population that will be enrolled in future clinical trials.
Challenges and Competitive Landscape in Advancing Combination Therapy for PTCL
Although focusing on combination therapy presents challenges, Affimed believes the potential benefits of combining AFM13 with AB-101 NK cells are worth pursuing. However, combining different drugs or therapies can be difficult due to varying dosing schedules, side effects, and interactions. Getting combination therapies FDA-approved and to market can also be more difficult, as the FDA requires extensive testing and data to demonstrate their safety and efficacy.
The competitive landscape in PTCL treatment may have also influenced the decision to abandon monotherapy and focus on combination therapy. Brentuximab vedotin (Adcetris), recombinant chimeric mAb directed against CD30, + chemotherapy and pralatrexate (Folotyn) are two examples of FDA-approved therapies for PTCL.
Adcetris and chemotherapy is the preferred induction therapy for CD30-positive PTCL, achieving an ORR of 87% and a CR of 68% without additional toxicity in a pivotal clinical trial. Folotyn was approved in 2009 for relapsed or refractory PTCL, with an ORR of 29%, CRR of 11%, and median DOR of 10.1 months. However, Folotyn is associated with significant toxicity and is typically reserved for patients who have failed other treatments.
Given the approval of these drugs and the crowded market for PTCL treatments, Affimed may have felt their monotherapy results were not strong enough to compete. The combination of AFM13 with AB-101 NK cells may offer a more compelling treatment option, as the AFM13-104 trial showed an ORR of 89% and a CRR of 59% in patients with relapsed or refractory Hodgkin lymphoma. While the results certainly cannot be extrapolated to PTCL, they suggest that combining AFM13 with NK cells may be a more effective treatment approach.
Challenges of Developing Combination Therapies in Cancer Treatment: Understanding Investor’s Negative Response to Affimed’s Decision to Abandon Monotherapy in PTCL.
Investors’ negative response to Affimed’s decision to abandon monotherapy in PTCL is understandable given the challenges associated with developing and gaining FDA approval for combination therapies. Combination therapies involve the use of two or more drugs or therapies in the treatment of a disease, with the aim of improving efficacy and/or reducing toxicity. While there have been some successful examples of combination therapy in cancer treatment, the development and FDA approval of combination therapies can be a long and difficult process.
One example of the challenges associated with developing combination therapies is the case of ipilimumab and nivolumab, which are both checkpoint inhibitors that have been approved by the FDA for the treatment of melanoma. While these drugs have shown promising results in combination, their development and FDA approval was a lengthy process that involved several clinical trials. In addition, the combination therapy has been associated with increased toxicity, which has limited its use in some patients.
Another example is the combination therapy of chemotherapy and radiotherapy in the treatment of head and neck cancer. While this combination has shown promise in some patients, there are still many unknowns about how the two therapies interact with each other, and the optimal timing and dosing of the two therapies is still being investigated.
In the case of PTCL, there have been some past attempts at combination therapy, but none have been widely adopted as standard treatment. For example, the combination of chemotherapy and radiation therapy has been used in the past, but this has been associated with significant toxicity and has not shown significant improvement in overall survival. In addition, the combination of chemotherapy and stem cell transplantation has been used in some patients, but this is a high-risk procedure that is not suitable for all patients.
Conclusion
In summary, Affimed’s decision to pivot from monotherapy to combination therapy for peripheral T-cell lymphoma is a strategic one intended to increase the likelihood of AFM13’s success. While the monotherapy results were promising, the combination approach is expected to be more effective and competitive. However, advancing combination therapy is a difficult process that requires extensive clinical trials and regulatory approvals. Additionally, achieving success with AFM13 and AB-101 NK cells in the face of Adcetris and chemotherapy in combination therapy is a long-shot. Affimed is starting from scratch to pursue a challenging goal, and its resources are limited. Investors are understandably cautious, as Affimed and AFM13 are still a long way from being market-ready, and the company will need to stretch its resources to achieve its objectives. It is worth noting that biotechnology stocks may trade under their cash value, but that does not necessarily make them undervalued or cheap. As long as Affimed remains active, it is likely to burn through at least $75 million annually and resort to dilutive capital-raising measures and debt. Although AFM13 for PTCL is Affimed’s primary and most-advanced focus, the company also has other prospects, such as AFM24. However, since these assets are in the preclinical or phase 1 stage, it is challenging to determine their value.
Analyst’s Disclosure: I/we have no stock, option or similar derivative position in any of the companies mentioned, and no plans to initiate any such positions within the next 72 hours. I wrote this article myself, and it expresses my own opinions. I am not receiving compensation for it (other than from Seeking Alpha). I have no business relationship with any company whose stock is mentioned in this article.
This article aims to provide informational insight and not personal investment advice. The information presented is intended to be factual, but readers are encouraged to independently verify the information and consider their own financial situation, risk tolerance, portfolio diversification, etc. before making an investment decision. Some of the articles cover biotechnology companies with limited or no revenue, which makes the stocks speculative and prone to volatility. While the prospects may appear attractive, it's important to keep in mind that the future is unpredictable and there is a potential for significant losses.
Seeking Alpha’s Disclosure: Past performance is no guarantee of future results. No recommendation or advice is being given as to whether any investment is suitable for a particular investor. Any views or opinions expressed above may not reflect those of Seeking Alpha as a whole. Seeking Alpha is not a licensed securities dealer, broker or US investment adviser or investment bank. Our analysts are third party authors that include both professional investors and individual investors who may not be licensed or certified by any institute or regulatory body.
