Sviatlana Barchan/iStock via Getty Images
Novo Nordisk (NVO) and Eli Lilly & Co. (NYSE:LLY), the global leaders in obesity medications, are now facing their first major competitor in China.
Suzhou-based Innovent Biologics (OTCPK:IVBIY) (OTCPK:IVBXF) gained regulatory approval last week for its obesity treatment, mazdutide—a significant milestone in China’s push to tackle rising obesity and diabetes rates through homegrown innovation.
Innovent first licensed mazdutide from Lilly (NYSE:LLY) in 2019, when the drug was in mid-stage testing. The Chinese company ran large clinical trials suggesting that in local patients, it appeared just as effective as Lilly’s (LLY) bestselling Zepbound in helping patients lose weight.
“The approval of mazdutide was mainly based on data from GLORY-1, a Phase 3 pivotal clinical study conducted in Chinese adults with overweight or obesity. The primary endpoint and all key secondary endpoints of the study were successfully achieved in 2024,” the company said.
(Innovent Biologics) 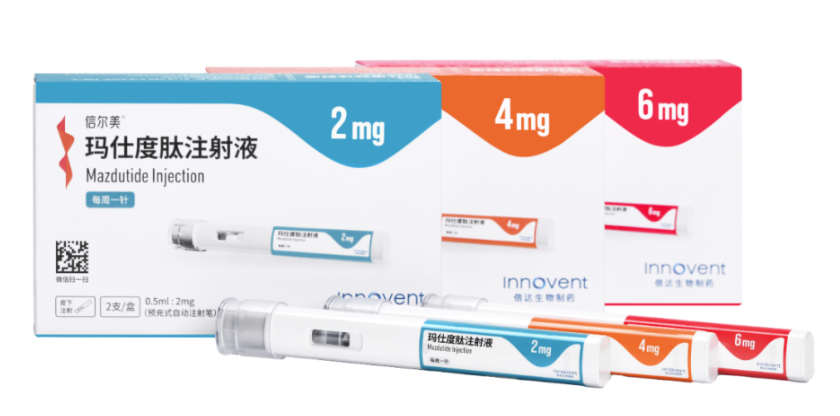
“Results showed that at weeks 32 and 48, the percentage of body weight reduction from baseline and the proportions of participants with a body weight reduction of ≥5%,≥10% and ≥15% in the mazdutide 4 mg group and mazdutide 6 mg group were superior to those of the placebo group.”
More on Novo Nordisk, Eli Lilly, etc.
- Eli Lilly: I’ve Learned Not To Fight Against The Market (Downgrade)
- Buy Eli Lilly While The Market Hesitates And Let Time Make You Dominate
- Eli Lilly: It Is A Bet On Human Longevity
- Dividend Roundup: Eli Lilly, Mastercard, AT&T, Cisco, and more
- Novo Nordisk taps WeightWatchers for Wegovy after exiting Hims & Hers deal