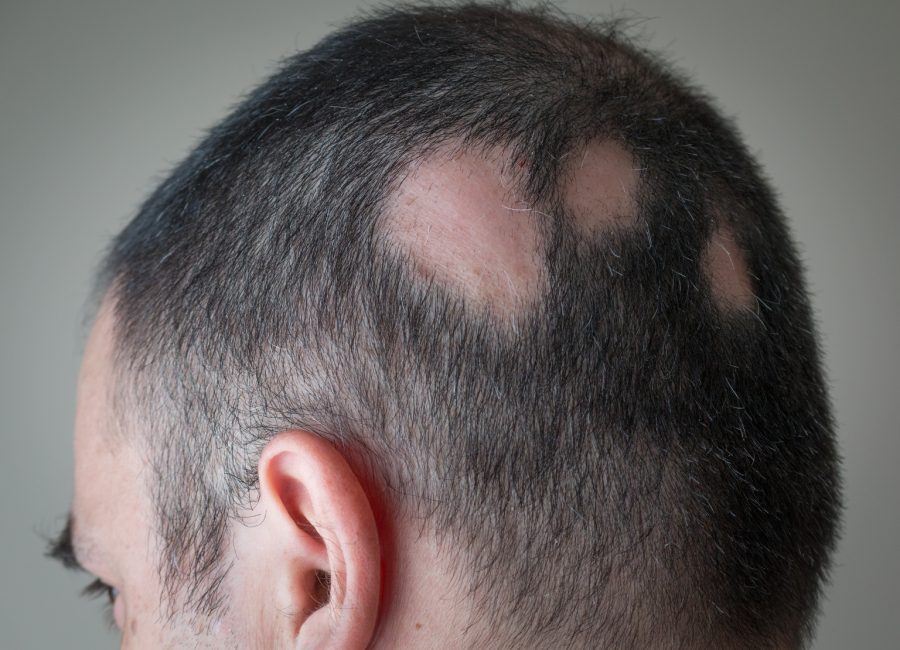
Eli Lilly (NYSE:LLY) and Incyte (NASDAQ:INCY) are set to seek a label expansion for their JAK inhibitor baricitinib, marketed as Olumiant for alopecia areata, after new results from a Phase 3 trial indicated its potential in adolescents with the hair loss condition.
Citing 52-week results from their BRAVE-AA-PEDS trial, the companies said that up to 54% of patients who received the once-daily, oral therapy experienced 80% or more scalp hair coverage, indicating successful hair regrowth.
The ongoing trial involved those aged 6 to under 18 years with severe AA, and the readout represented its first two cohorts, targeted at more than 400 adolescents aged 12 to under 18 years.
Noting that trial participants had an average scalp hair loss of 89% at the start of the study, the duo said about 41% and 26% of subjects experienced 90% or more scalp hair coverage, witnessing near-complete scalp hair growth in response to baricitinib 4 mg and 2 mg, respectively.
There were no new safety signals in BRAVE-AA-PEDS, the companies added, noting that the results build on a prior readout on 32-week data from the study as presented at the 2025 American Academy of Dermatology Annual Meeting in March.
With Olumiant currently indicated in the U.S. for adults with AA, Eli Lilly (NYSE:LLY) and Incyte (NASDAQ:INCY) plan to share the latest results with global regulators in pursuit of a label expansion for the drug in adolescents. Enrollments for the next cohort of the trial targeting children aged 6 to under 12 are expected to start in 2026.