Summary:
- Shares of Acadia Pharmaceuticals Inc. have been stuck in a range for the last two years.
- The approval and launch of Daybue come at the right time – when Nuplazid is not growing anymore.
- Growth reacceleration, the company potentially reaching profitability next year and upcoming pipeline catalysts put Acadia in a good position to finally start delivering shareholder value.
RomoloTavani
Shares of Acadia Pharmaceuticals Inc. (NASDAQ:ACAD) have been stuck in a range for more than two years. More specifically, since the major regulatory setback – when the FDA rejected to approve Nuplazid for the treatment of dementia-related psychosis. The company has decided to switch its focus to the next-generation candidate ACP-204 for the treatment of Alzheimer’s disease psychosis, on Nuplazid as an adjunct treatment for schizophrenia and it has recently secured FDA approval for Daybue (trofinetide) for the treatment of Rett syndrome.
The company also dialed down the commercial spending on Nuplazid, and sales growth has all but disappeared in the last few quarters and the company guided for a flat to slightly higher year. Nuplazid in the currently approved indication (Parkinson’s disease psychosis) is probably going to be a cash cow for the company going forward and Acadia will have to rely on Daybue in the near and medium term, Nuplazid in schizophrenia (if successful in the phase 3 trial next year) in the medium term, and longer term on ACP-204 as an improved version of Nuplazid and on business development.
With cash flow breakeven and profitability around the corner, a new growth phase driven by Daybue, and pipeline maturation, Acadia looks well-positioned to finally start delivering shareholder value in the following quarters and years.
Timely approval of Daybue as Nuplazid stops growing
The approval of Daybue is really timely from a revenue growth standpoint as the company expects Nuplazid sales to be in the $520 million to $550 million range in 2023 and that would represent less than 1% growth at the low end of the range and 6% growth at the high end of the range. And this comes on top of very modest 7% growth in 2021.
Nuplazid has been a profitable product since 2019 (in terms of commercialization costs, not for the company as a whole) and the company has reduced spending over the last two years to better manage the cash flows.
Daybue became commercially available this month and it should provide a big lift to the overall revenue growth rates in the following quarters. Rett syndrome is an orphan indication, and that is how management justified the massive annual list price of $575,000 per patient. When discounts are added and when compliance and reduced dosing are considered, the company expects the net price to settle at around $375,000 per patient per year.
The company estimates the prevalent U.S. population is between 6,000 and 9,000 patients with around 4,500 diagnosed patients (Acadia only holds the rights for North America). If we take the company’s estimated net price, the total addressable market, or TAM, is between $2.25 billion and $3.4 billion.
However, discontinuation rates were on the higher side in clinical trials and patients taking Daybue experienced very high diarrhea rates (82% of patients) and vomiting (29%). Of the 93 patients enrolled in the Daybue arm in the phase 3 trial, 76 patients made it out to 12 weeks, and of the 187 patients that were enrolled in the trial, the company reports there are approximately 80 patients left in the open-label extension study (placebo-treated patients in the randomized study transitioned to Daybue in the open-label extension study). So, that’s a relatively high attrition rate, and we could say that of 100 patients that start taking Daybue today, it is reasonable to assume about 40 will stay on the drug for 12 months or longer.
However, the company does believe it can manage gastrointestinal side effects better in the real world, and this could lead to better patient retention in the commercial setting, but I do not believe Daybue’s profile and the nature of the disease warrant high penetration rates. Getting to 15-20% peak market penetration would be a reasonable goal for Acadia and a very reasonable outcome for investors, as it would translate to approximately $350 million in annual sales at the low end of the estimated prevalence (6,000 patients) and penetration (15%) ranges, and nearly $700 million at the high end of the prevalence and (9,000 patients) and market penetration (20%) ranges.
The good news for Acadia is that expectations this year are very modest. The Street revenue consensus for 2023 is only $559 million, which implies as low as $9 million in Daybue sales if we take the high end of the guidance range for Nuplazid and nearly $40 million in Daybue sales if we take $520 million for Nuplazid. The transition of the 80 patients from the open-label extension study to the commercial drug alone should provide a nice start for Daybue in the following months.
Nuplazid as an adjunct treatment of schizophrenia and ACP-204 are the main pipeline assets
In the long term, Daybue and Nuplazid in PDP alone are likely insufficient for meaningful value creation. That is where the pipeline comes in. Granted, there are only two mid/late-stage shots on goal, but both are potentially very valuable.
The results from the second trial of Nuplazid as an adjunct treatment of negative symptoms of schizophrenia are expected in the first quarter of 2024. This could be a more valuable indication than PDP, but I also wonder what the access strategy will be given Nuplazid’s very high price tag for this population. In any case, this indication could lead to Nuplazid sales doubling or more than doubling by the end of the decade. The first study was successful, but the benefit was not exactly robust, and I would not say the probability of success is on the high side. The primary endpoint was barely met with a p-value of 0.043 for all doses combined, but the 34mg dose that the company took forward did demonstrate better efficacy.
And ACP-204 could be a real winner but carries similar or perhaps even greater development risk. Acadia pivoted from Nuplazid to ACP-204 after failing to convince the FDA to approve Nuplazid for the treatment of Alzheimer’s disease psychosis, and the company says ACP-204 is an improved version of Nuplazid, specifically on the safety side where it believes it can reduce or eliminate the key safety issue of Nuplazid – QT prolongation. The company also believes it can push ACP-204 more aggressively and that it can achieve efficacy faster – it gets to steady-state levels twice as fast as Nuplazid.
Reasonable valuation and renewed growth and upcoming profitability are a good recipe for value creation
I think Acadia can deliver decent gains in the following quarters and years. The launch of Daybue should lead to a reacceleration of topline growth and the company should reach cash flow breakeven within a few quarters and profitability (on a quarterly basis) sometime next year.
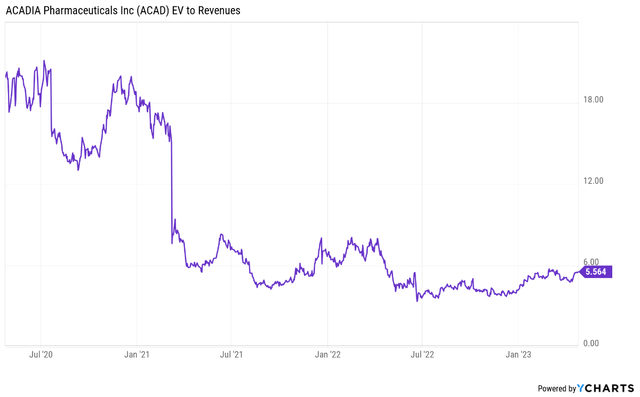
If we add the stock trading at an enterprise value/revenue ratio near historical lows (around 5.5 as of this writing) and the upcoming clinical catalyst for Nuplazid in early 2024 and the likely fast progression of ACP-204 through the clinic, we get a decent setup for the next few quarters. If Daybue lives up to current expectations and revenue growth accelerates to a 25% to 30% range, I believe it is reasonably likely that the stock will revisit last year’s valuation highs of EV/revenue of around 8 and that it should easily exceed that level if Nuplazid is successful in the schizophrenia phase 3 trial next year.
The risks during this period are Daybue not reaching or exceeding the current consensus estimates, Nuplazid failing in the schizophrenia trial next year and ACP-204 not living up to expectations of an improved profile compared to Nuplazid and failing to produce a strong enough benefit in Alzheimer’s disease psychosis patients. I do believe the clinical risks are largely reflected in the current valuation and that Acadia Pharmaceuticals Inc. could produce decent returns even without the schizophrenia indication or without ACP-204. However, the long-term upside, in that case, is far more limited, and Daybue would need to be a resounding success in that case.
Analyst’s Disclosure: I/we have no stock, option or similar derivative position in any of the companies mentioned, and no plans to initiate any such positions within the next 72 hours. I wrote this article myself, and it expresses my own opinions. I am not receiving compensation for it (other than from Seeking Alpha). I have no business relationship with any company whose stock is mentioned in this article.
Seeking Alpha’s Disclosure: Past performance is no guarantee of future results. No recommendation or advice is being given as to whether any investment is suitable for a particular investor. Any views or opinions expressed above may not reflect those of Seeking Alpha as a whole. Seeking Alpha is not a licensed securities dealer, broker or US investment adviser or investment bank. Our analysts are third party authors that include both professional investors and individual investors who may not be licensed or certified by any institute or regulatory body.
I publish my best ideas and top coverage on the Growth Stock Forum. If you’re interested in finding great growth stocks, with a focus on biotech, consider signing up. We focus on attractive risk/reward situations and track each of our portfolio and watchlist stocks closely. To receive e-mail notifications for my public articles and blogs, please click the follow button. And to go deeper, sign up for a free trial to Growth Stock Forum.