Naeblys
- Phase 1b data on Johnson and Johnson’s experimental CAR T-cell therapy JNJ-4496 found a high complete response rate at the recommended phase 2 dose in patients with large B-cell lymphoma
- Among evaluable patients at that dose, a 75%-80% complete response rate was observed.
- Patients enrolled in the study had not been previously treated with a CAR-T therapy.
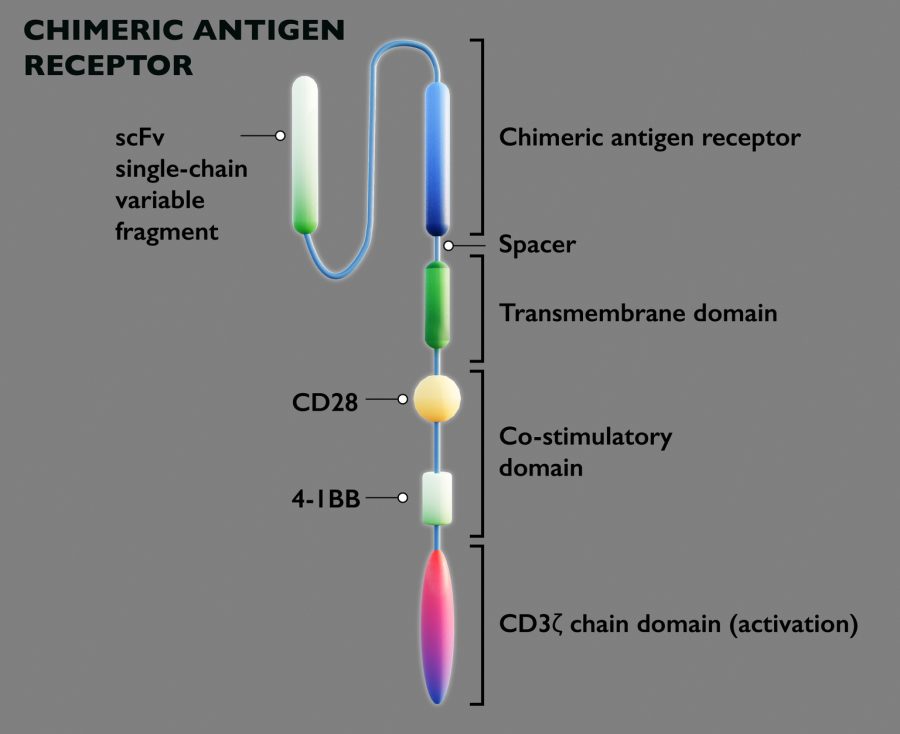
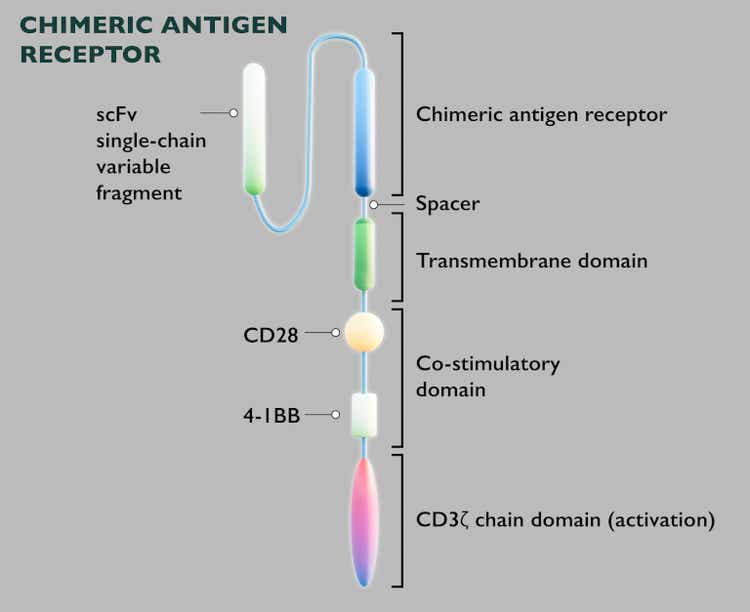
- JNJ-4496 targets CD19 and CD20 antigens, two cell surface proteins frequently found on malignant B-cells.
More on J&J
- Johnson & Johnson (JNJ) Presents at Goldman Sachs 46th Annual Global Healthcare Conference Transcript
- Johnson & Johnson: Relative Stability In A Wild 2025 Stock Market
- Johnson & Johnson (JNJ) Presents at the Bernstein’s 41st Annual Strategic Decisions Conference (Transcript)
- Short bets on S&P 500 Healthcare sector ease in May; MRNA stays most shorted stock
- J&J touts phase 1 data for bleximenib for acute myeloid leukemia