tumeyes/iStock via Getty Images
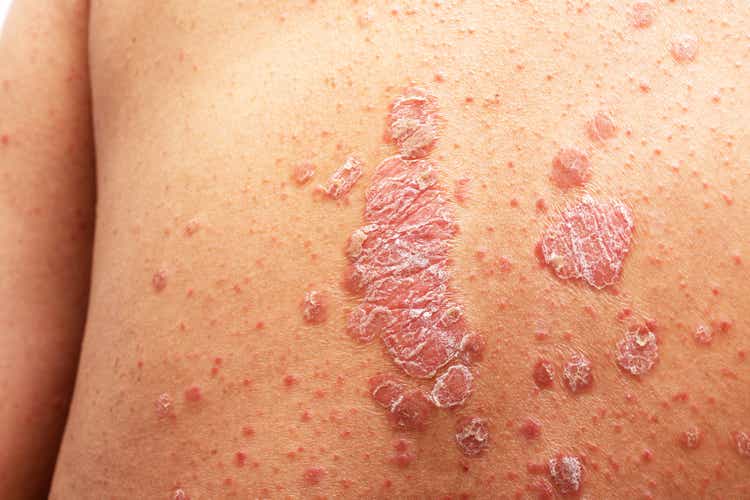
- A phase 3 trial of Johnson & Johnson’s (NYSE:JNJ) icotrokinra for plaque psoriasis met its co-primary endpoints: Psoriasis Area and Severity Index (PASI) 90 and Investigator’s Global Assessment (IGA).
- Topline results from the ICONIC-LEAD study showed that at week 16, patients given oral icotrokinra, 64.7% demonstrated an IGA scores of 0/1 — clear or almost clear skin — and 49.6% achieved PASI 90, compared to 8.3% and 4.4% on placebo, respectively.
- At week 24, 74.1% of patients of those on icotrokinra achieved IGA scores of 0/1, while 64.9% achieved PASI 90.
- J&J said that icotrokinra is the first targeted oral peptide that selectively blocks the IL-23 receptor.
- The healthcare giant is partnered with Protagonist Therapeutics (NASDAQ:PTGX) on icotrokinra. Under a collaboration agreement, Protagonist will receive $165M in milestone payments, which it expects to receive in Q1 2025.