Summary:
- Biogen, Eisai, and Eli Lilly’s Alzheimer’s drugs have shown little efficacy, particularly in non-APOE4 carriers.
- The companies recast the data to suggest non-carriers benefitted more from their drugs, but in reality, non-carriers benefitted least as they often have little to no amyloid in their brains.
- The drugs may also be ineffective for women with the APOE4 gene due to high levels of amyloid and oxidative stress.
- Serious safety concerns in APOE4 carriers could increase as the drugs are used in larger populations.
Olga Donchuk/iStock via Getty Images
The two main tests for a drug are efficacy and safety. As I have written before, Eisai (OTCPK:ESALF) and Biogen’s (BIIB) Leqembi and Eli Lilly’s (NYSE:LLY) donanemab largely fail on both accounts. In this article, I am going to expand on my previous analysis and look at a group that I missed in terms of lack of efficacy but others did not: women. The safety issues are more likely to be the Achilles heel for both companies, but the efficacy issues could cause some troubles as well. In this respect, Eli Lilly is more at risk of a substantial downturn than Biogen. Over the course of about two and a half years, its stock value has gone from $200 to $460. The financial success of its anti-diabetes drug Mounjaro has certainly contributed to Eli Lilly’s soaring stock price, but this level may not be sustainable, and even less so as problems (or if problems) arise with donanemab. My previous sell recommendations have been either inaccurate or premature, but Eli Lilly is reaching a level that few pharmaceutical companies have ever reached and therefore (or maybe because) of its recent history, caution seems prudently advised.
And now for a discussion of who (minimally) benefits from these drugs and who doesn’t and why.
Non-APOE4 Carriers
Both Biogen and Eisai and Eli Lilly once acknowledged that their drugs had no appreciable effect on non-APOE4 carriers:
BAN2401/lecanemab/Leqembi
Did APOE4 affect response to treatment? The subgroup analysis said yes…Carriers on the highest dose had less cognitive impairment at 18 months than the noncarriers or the group overall. On ADCOMS [Alzheimer’s Disease Composite Score], for example, where the highest dose group declined 30 percent less than placebo, APOE4 carriers declined 63 percent less and noncarriers only 7 percent less (source of quote).
Donanemab
Relationship between amyloid reduction and iADRS [Integrated Alzheimer’s Disease Ratings Score]…were significant in APOE4 carriers, but not in participants without APOE4 (source of quote).
Biogen and Eisai and Eli Lilly, though, in announcing final results completely turned this around arguing that non-carriers benefitted significantly more from their drugs than carriers.
Biogen (and Eisai) accomplished this by comparing the decline of non-carriers on lecanemab against the combined placebo decline of non-carriers and carriers rather than against the placebo decline in just non-carriers. Non-carriers on the drug declined by .75 points less than the combined placebo, but only by about .15 points less than non-carriers on placebo (as measured by CDR-SB scores: Clinical Dementia Rating-Sum of Boxes).
Eli Lilly appeared to do the same thing, although it threw in a new wrinkle by linking the differential effects of donanemab to tau levels (the following numbers represent the difference from placebo as measured by CDR-SB scores; heterozygotes have one copy of the APOE4 gene, homozygotes have two copies of the APOE4 gene):
Combined tau population (p. 35)
- Non-carriers -.76
- Heterozygotes -.73
- Homozygotes -.41
Lecanemab for total population (p. D57)
- Non-carriers -.75
- Heterozygotes -.5
- Homozygotes +.28
This apparent sleight of hand made it look like non-carriers benefitted most from donanemab and lecanemab. But in actuality, non-carriers were the group who benefitted least from these drugs.
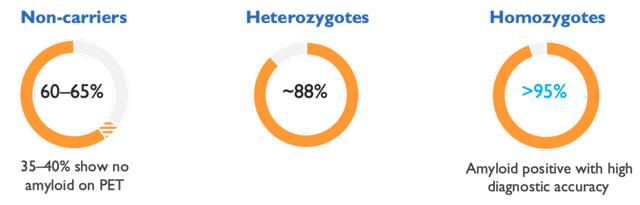
The reason why anti-amyloid drugs don’t help non-carriers is that many non-carriers have little to no amyloid in their brain to begin with.
In essence, anti-amyloid antibody drugs are like using a hammer when a screw driver is needed instead.
Women
Women with the APOE4 gene (or genes) tend to have both more amyloid and tau tangles than men with the APOE4 gene. One would assume that they would benefit more from anti-amyloid antibodies than men, but this does not appear to be the case (gantenerumab results). For lecanemab, women declined by 12 percent on CDR-SB scores versus 43 less for men (article). For Eli Lilly, in the high tau group (over 60 percent who were women), donanemab slowed down the progression of the disease on the same measure by 16.8 percent (pp. 7, 9).
Whereas the problem is too little amyloid in non-carriers the problem may be too much amyloid in women (or more precisely too much oxidative stress). In general, women tend to have a more active innate immune system than men, leading to the greater production of the nitro-oxidant peroxynitrite. This may be good for fighting off various infections, but bad for the development of various autoimmune diseases and Alzheimer’s disease. Women with Alzheimer’s disease carrying the APOE4 gene have more oxidative stress and neuroinflammation than their male counterparts (as a whole), such that removing amyloid has less effect. To put it another way, the percentage of the disease caused by amyloid decreases when the oxidative stress caused by other factors increases (notably in women with the APOE4 gene). Thus the Goldilocks’ effect, anti-amyloid drugs are not effective in those with too little or too much amyloid.
Safety
In a larger population, the number of brain bleeds and brain swelling that can lead to death are likely to increase substantial from phase 3 clinical trials. Leqembi and donanemab (which will most likely be approved by the FDA near the end of this year) are certainly not the only drugs that can cause death. But in a particular vulnerable population that cannot really give informed consent, that is a heavy burden to place on caregivers who are desperate for an effective treatment for the disease.
Concluding Remarks
I think it is more likely that Medicare will change its mind regarding covering these drugs as safety and efficacy concerns mount than the FDA is to rescind approval. Both more doctors and more caregivers may become increasing less willing to subject their patients and their loved ones to burdensome treatments that likely have no appreciable effect on a large portion of the population. At best, these drugs provide a minimally clinically significant effect mainly in male APOE4 carriers, with potentially very serious side effects for all APOE4 carriers. Should things go south, Biogen’s stock value could drop modestly further and Eli Lilly’s stock value could drop substantially (despite its strong product line).
The amyloid era of Alzheimer’s disease was almost over until Biogen and Eisai discovered that APOE4 carriers on the highest dose of aducanumab for a longer period of time were showing some signs of slower disease progression. Some Alzheimer’s organizations and researchers who have touted the amyloid hypothesis for years feel vindicated by its revival. But the answers for Alzheimer’s disease are going to come from elsewhere and hopefully soon.
Editor’s Note: This article discusses one or more securities that do not trade on a major U.S. exchange. Please be aware of the risks associated with these stocks.
Analyst’s Disclosure: I/we have no stock, option or similar derivative position in any of the companies mentioned, and no plans to initiate any such positions within the next 72 hours. I wrote this article myself, and it expresses my own opinions. I am not receiving compensation for it (other than from Seeking Alpha). I have no business relationship with any company whose stock is mentioned in this article.
Seeking Alpha’s Disclosure: Past performance is no guarantee of future results. No recommendation or advice is being given as to whether any investment is suitable for a particular investor. Any views or opinions expressed above may not reflect those of Seeking Alpha as a whole. Seeking Alpha is not a licensed securities dealer, broker or US investment adviser or investment bank. Our analysts are third party authors that include both professional investors and individual investors who may not be licensed or certified by any institute or regulatory body.