Summary:
- Obesity affects a significant percentage of adults around the world and is linked to various serious health conditions.
- There has been a lot of hype about GLP-1 drugs as they are considered the definitive solution to obesity.
- Two companies dominate the market for these drugs and their valuations have skyrocketed in recent years.
- The current valuations are not sustainable and there exist numerous adverse factors that could lead to a substantial decline in the stock price.
imyskin/iStock via Getty Images
Obesity affects nearly 42 percent of American adults and as reported by a report from the World Obesity Atlas, more than half of the global population – over 4 billion people – will be overweight or obese by 2035 with related health costs topping $4 trillion per year. Individuals grappling with overweight or obesity confront an elevated vulnerability to an array of serious health afflictions. These health hazards encompass an increased risk of all-cause mortality, hypertension, dyslipidemia and elevated triglyceride levels. They face a higher likelihood of developing type 2 diabetes, coronary heart disease, strokes, gallbladder disease and osteoarthritis. Respiratory challenges, including sleep apnea, become more prevalent and there is increased risk of growing various forms of cancer. Furthermore, mental health bears the brunt, as conditions like clinical depression, anxiety, and related disorders become more common. Overweight people also grapple with bodily discomfort and limitations in physical functioning, underscoring the multifaceted nature of the health risks associated with excess weight and its impact on the quality of life.
From an economic perspective, ending obesity once and for all has the potential to yield tremendous cost savings for healthcare systems.
In light of these premises, a drug possessing the potential to effectively treat a condition that affects such a substantial portion of the population and the economy and at the same time be safe to use, is bound to become a huge blockbuster.
Game changers
A new class of drugs has emerged in the last few years that has the potential to become a global revolution, short only of an eternal youth pill: glucagon-like peptide 1 (GLP-1) agonists.
Glucagon-like peptide-1 (GLP-1), a hormone produced in the small intestine, plays a pivotal role in glucose regulation. Its functions encompass initiating insulin secretion and diminishing glucagon release, resulting in lowered glucose levels. Additionally, GLP-1 moderates the speed of stomach emptying, ensuring a gradual glucose release into the bloodstream. This mechanism can lead to enhanced blood sugar management in individuals utilizing these drugs. Furthermore, GLP-1 holds the potential to impact brain centers that regulate appetite, potentially curbing hunger.
GLP-1 agonist medications present variable effects dependent on dosage and type and typically involve the following outcomes: a deceleration of gastric emptying, resulting in diminished post-meal glucose levels; suppression of appetite; heightened satiety and the promotion of growth in pancreatic beta cells responsible for insulin production, thus aiding in glucose regulation enhancement and weight reduction for individuals with diabetes. Research suggests potential favorable influences on cardiovascular health, encompassing the mitigation of risk factors such as high blood pressure and elevated cholesterol levels.
The next trillion dollar companies?
Analysts at Morningstar predict that in the next ten years the global market for GLP-1 agonists could reach sales of $86 billion annually, but I guess these figures are related to diabetes and off-label prescriptions only; in fact, according to the World Health Organization, by 2030 there will be one billion obese and two billion overweight individuals, so the addressable market for these drugs will be immense.
Currently the market is dominated by two companies: Novo Nordisk (NVO) – headquartered in Denmark – and Eli Lilly (NYSE:LLY), residing in Indiana, USA. In 2021, Novo Nordisk got approval from the F.D.A. to market his compound, called Semaglutide, for the treatment of obesity with a weekly injection and the drug was named Wegovy. Eli Lilly’s compound, Tirzepatide, is a GLP-1 agonist of the second generation and is currently prescribed for diabetes management only, but the company has already filed the request to receive approval for the treatment of obesity as well. It brags that its drug, named Mounjaro, delivers better results than any other competitor, with weight losses of up to 25% body weight.
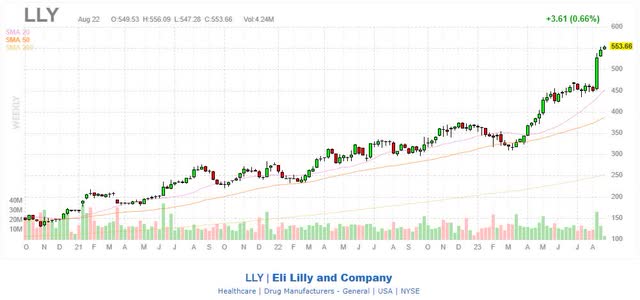
Amidst soaring sales and the anticipation of continued growth, investors have propelled the share prices of these two companies over the recent years. Both Novo and Lilly now boast valuations threefold greater than their counterparts, with Lilly’s market capitalization surpassing 500 billion dollars, making it the world’s most valuable pharmaceutical company.
Time to sell
In my view, the current price has seemingly attained its zenith and a lot of uncertainties regarding future sales of these drugs should be accounted for; let’s discuss them.
Weight regain
Studies highlight that individuals often regain lost weight once these medications are no longer taken, and may even add some more.
To avoid experiencing cyclical patterns of fitness and obesity, individuals often need to commit to lifelong medication and this comes with specific risks and recurring costs.
High costs
Eli Lilly’s Mounjaro comes with a price tag of up to $250 per week for those without private insurance coverage and a prescription, an expense that only few affluent individuals can afford.
Several insurance providers in the United States are declining coverage for these treatments, categorizing them as non-essential or cosmetic medications out of vanity.
If this becomes common practice among insurers, those hefty sales figures could evaporate in a few months.
Side effects and potential regulation
Mounjaro’s official site lists a series of known side effects, that can be applied to all the other drugs in the same class; besides the most common that include nausea, diarrhea, decreased appetite, vomiting, constipation, indigestion and abdominal pain, the list includes less common consequences such as pancreatitis, low blood sugar and serious allergic reactions – conditions that may require immediate medical attention – kidney failure, gallbladder pain, jaundice, reduced vision and severe stomach problems.
Moreover, the company adds that they don’t know whether the drug can safely be taken by people who had suffered from pancreatitis in the past.
As stated by the National Pancreas Foundation each year in the U.S. nearly 220,000 people are afflicted with acute pancreatitis and more than 80,000 people are diagnosed with chronic pancreatitis so if we add up the numbers, it is likely that in the U.S. alone there exist at least 10 million people who have suffered from pancreatitis at one time in their life and should think twice before beginning a treatment with these drugs.
This is the list of known problems; GLP-1 drugs are also associated with other reported and under scrutiny issues that are even more scary.
In July 2023, the Medicines and Healthcare products Regulatory Agency of the U.K. revealed to Reuters its ongoing examination of safety information concerning this drug category. This comes in response to patient accounts of experiencing thoughts related to self-harm or suicide. Correspondingly, the European Medicines Agency also declared its intention to conduct a comparable review.
The results are still inconclusive, but there are ongoing studies regarding the potential connection between these drugs and an the risk of developing thyroid and pancreatic cancer.
These drugs have also been associated with major loss of muscle mass and bone density, definitely not a desirable outcome if you’re wishing to be fit.
As the comprehensive safety profile of a product unfurls over the course of months and even years during its life on the market, there exists the potential for regulatory bodies such as the FDA, EMA and other national agencies to intervene by enforcing regulations aimed at limiting the use cases of these drugs.
Unknown mechanisms of action
Due to their recent discovery and limited time on the market, a thorough understanding of these drugs’ mechanism of action is still lacking. Basically scientists stumbled upon the fact that subjecting the brain to a naturally occurring hormone at unprecedented levels led to weight loss, yet the metabolic and biochemical factors contributing to this result remain unclear.
In the words of Dr. David D’Alessio, chief of endocrinology at Duke University, who consults for Eli Lilly:
Everyone would like to say there must be some logical explanation or order in this that would allow predictions about what will work; so far there is not.
Moreover, the drug is going into cells in the body where it is not supposed to go; Dr. Seeley, who has consulted for Novo Nordisk and Eli Lilly, among others, on the matter declared:
They are not just going to areas thought to control overeating; If you were designing a drug, you would say that’s a bad idea.
The long-term effects of a drug that goes around freely in the body and whose mechanisms of action are still unclear, are anyone’s guess.
iStock
Shortages
Although demand for these drugs is outpacing available supplies and investors are cheering, it also means that sales plateaued and the companies need to spend large sums of money to expand their manufacturing facilities; until these plants are complete and fully operational there will be no further increase in sales.
Competition and patents
An old saying goes: “Becoming number one is easier than remaining number one”; according to Morningstar analysts, Amgen (AMGN), Pfizer (PFE) and other biopharma firms could begin launching their own GLP-1-based obesity drugs as early as 2025, taking away from Novo and Lilly roughly one quarter of the market by 2032.
It must also be considered that while patents remain valid in the US for a period of 20 years following the drug’s invention, the process of conducting sufficient testing and obtaining approval from the U.S. Food and Drug Administration (FDA) can extend up to eight years. Therefore, after receiving FDA approval, the overall timeframe usually spans from 7 to 12 years. Once the patent reaches its expiration, generic pharmaceutical companies come into the picture. Legislation in other countries may also cause some trouble to Novo and Lily. In Brazil a federal court has already denied a patent extension for Semaglutide and in China generics will be available as soon as 2026. It is possible that other nations may allow for the manufacturing of generics before patents expire in the U.S. and, given the high prices that these pharmaceuticals command, drug smuggling and counterfeit products may appeal to criminal organisations and subtract a significant chunk of revenue from these companies.
Alternative drugs
While there is a lot of hype around these pharmaceuticals as if they were the only game in town, the general public forgets that there are already on sale much cheaper drugs and supplements that can yield similar weight loss effects, such as Metformin and Berberine; the latter, a compound extracted from plants and that has been used in Asian medicine for centuries, has shown promising results in reducing blood sugar levels and body fat. It is a supplement that can be bought without any prescription and costs around $40 per month. Metformin is a drug used in the treatment of diabetes and is extremely cheap: without insurance, it will set you back for just $20 a month; the molecule has been around for decades: it was discovered in 1922, introduced as a medication in France in 1957 and in the United States in 1995. It needs a prescription for type 2 diabetes but doctors have been prescribing it off-label for years. It has been thoroughly tested and it has been conclude that it is extremely safe if taken as directed and although it cannot work miracles, shedding 10 to 15 pounds (5 to 7 Kg) is within reach for anyone. Combine that with a healthier diet, with less processed foods, sugar and alcohol, add some light walking every day and the weight loss can become even more significant. It may still not amount to the 25% body weight that Mounjaro promises, but the results will indeed be satisfactory, without risking breaking the bank or playing guinea pig.
Unsustainable valuation and final remarks
Novo Nordisk is currently selling at a price 43 times the earnings; Eli Lilly has a price-to-earnings ratio around 77 and a price-to-book ratio above 40; these valuations reflect a significant degree of investor enthusiasm in the companies’ prospects and guidance. However, the momentum could wane in the near future and Mr. Market might come to the realization that the aforementioned risk factors could emerge and take a toll on the stock price sooner or later. In my view, shareholders should set greed apart and collect their profits as I reckon that there is a significant possibility that the stock price of Eli Lilly and Novo Nordisk could decline by as much as 30% before the end of 2023.
Analyst’s Disclosure: I/we have no stock, option or similar derivative position in any of the companies mentioned, and no plans to initiate any such positions within the next 72 hours. I wrote this article myself, and it expresses my own opinions. I am not receiving compensation for it (other than from Seeking Alpha). I have no business relationship with any company whose stock is mentioned in this article.
Seeking Alpha’s Disclosure: Past performance is no guarantee of future results. No recommendation or advice is being given as to whether any investment is suitable for a particular investor. Any views or opinions expressed above may not reflect those of Seeking Alpha as a whole. Seeking Alpha is not a licensed securities dealer, broker or US investment adviser or investment bank. Our analysts are third party authors that include both professional investors and individual investors who may not be licensed or certified by any institute or regulatory body.