Summary:
- Positive Phase 3 data from Bavarian Nordic’s chikungunya virus (CHIKV) vaccine candidate, PXVX0317, reinforces bullish view on Valneva’s VLA1553 due to its superior clinical profile.
- Valneva’s VLA1553 demonstrated impressive seroresponse rates and durability, with future data releases from Bavarian Nordic’s additional Phase 3 trial expected to provide a comprehensive comparison between the two vaccines.
- Valneva has submitted a regulatory application to Health Canada for VLA1553 approval and received Fast Track.
- We continue to be a big fan of Valneva’s robust vaccine pipelines and maintain a buy rating.

PeopleImages/iStock via Getty Images
Key update: Bavarian Nordic’s data release adds to our conviction around Valneva’s VLA1553
We believe the near-term investor focus revolves around the Chikungunya Vaccine, where the PDUFA date is expected in August 2023, which is on track, and approval should provide a meaningful stock impact of 10-20% considering its $100-150m peak sales expectation.
The recent announcement of positive Phase 3 results from Bavarian Nordic’s chikungunya virus (CHIKV) vaccine candidate, PXVX0317, provides compelling evidence to reinforce our bullish stance on Valneva’s (NASDAQ:VALN) CHIKV vaccine, VLA1553, considering its superior clinical profile compared to PXVX0317 (more robust and persistent seroresponse). PXVX0317’s data revealed a neutralizing antibody seroresponse rate of 87% by day 22 and 82% by day 15 in adults aged 65 and above, which is encouraging. The safety profile of PXVX0317 also appears robust, with comparable tolerability observed across treatment and placebo groups.

Structure of CHIK-VLP (Research gate)
Of note, CHIKV VLP (PXVX0317) is a VLP-based vaccine in phase 3 development against chikungunya disease, where the phase 3 trial design is a multi-centered randomized controlled trial evaluating the safety and immunogenicity of the drug over 3,000 healthy individuals (aged 12-64).
Our conviction in VALN’s VLA1553 is strengthened by its superior clinical profile, as recently published in Lancet. The vaccine demonstrated impressive 98.9% seroresponse rates by day 29 in adults 18 and older, reaching 100% in individuals 65+, and showcasing durability through one year. Despite the more rapid onset observed with PXVX0317, VLA1553’s ability to induce a more robust and persistent seroresponse holds significant merit.
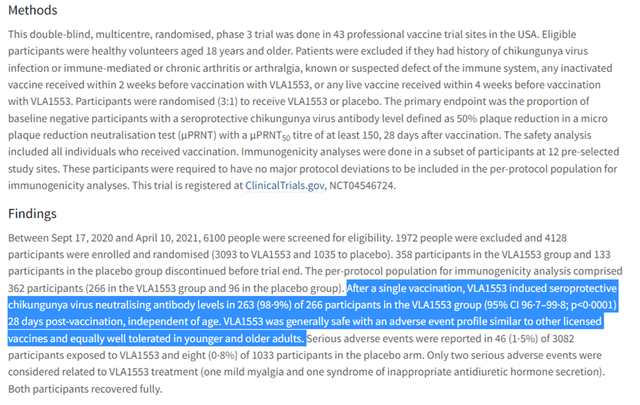
Lancet publication (Lancet publication)
Future data releases from Bavarian Nordic’s additional Phase 3 trial in the 12 to 64 age group, expected in Q3 2023, will provide a more comprehensive comparison between the two vaccines. Yet, with VLA1553’s studies indicating strong seroresponse rates at day 14 and a PDUFA date in August 2023, VALN’s candidate seems to be the fastest horse in the race. Ultimately, the key focal point will be the February 2024 ACIP meeting and vote, where VLA1553 remains the only CHIKV vaccine candidate under discussion.
Chikungunya Vaccine VLA1553 Filed For Approval in Canada
Valneva recently disclosed that it had submitted a regulatory application to Health Canada seeking approval to market VLA1553, its single-dose chikungunya vaccine, for individuals aged 18 and older. If Valneva’s application is accepted, Health Canada will outline a potential approval timeline.
VLA1553 has already received Fast Track and Breakthrough Therapy Designations in the US and PRIME designation in Europe. Of note, a study of VLA1553 in Brazilian adolescents is underway, with key results anticipated by mid-2023, potentially backing future submissions for adolescent use and licensure in Europe and Brazil. More submissions are expected for VLA1553 during 2023. It’s noteworthy that Valneva may qualify for a priority review voucher (PRV), possibly valued at around $100M, based on past PRV sales.
Risks
-
Regulatory Risk: The success of Valneva’s pipeline depends heavily on regulatory approval. Any unexpected delays, additional data requirements, or rejections from regulatory bodies could impact the company’s revenue and future growth prospects.
-
Clinical Trial Risk: Valneva’s products in development are subject to the results of clinical trials. If these trials do not produce the desired results or are discontinued for any reason, the investment in these products may not yield a return.
-
Market Acceptance and Competition Risk: The success of Valneva’s products also depends on acceptance by physicians, patients, and the medical community. If competing products prove more effective, safer, or less expensive, Valneva’s sales could suffer.
-
Supply Chain and Manufacturing Risk: Any disruptions in Valneva’s supply chain or manufacturing process, such as supply shortages or manufacturing difficulties, could negatively affect the company’s ability to meet the demand for its products, impacting revenues.
Conclusion
Net-net, in light of Valneva’s strong first-quarter performance in 2023 and the promising outlook for both its current products and development pipeline, we maintain a buy rating for the company. Despite a setback in the Lyme vaccine’s Phase 3 trial, we remain optimistic about its potential and expect approval by 2026. The anticipated approval of the Chikungunya Vaccine in August 2023 should positively impact the stock value, especially after we have seen the underwhelming data of the competitor product, CHIKV VLP’s (PXVX0317), and we don’t expect a meaningful delay in approval. Moreover, the management’s interest in potential in-licensing presents opportunities for portfolio diversification, adding further excitement to Valneva’s prospects.
Editor’s Note: This article discusses one or more securities that do not trade on a major U.S. exchange. Please be aware of the risks associated with these stocks.
Analyst’s Disclosure: I/we have no stock, option or similar derivative position in any of the companies mentioned, and no plans to initiate any such positions within the next 72 hours. I wrote this article myself, and it expresses my own opinions. I am not receiving compensation for it (other than from Seeking Alpha). I have no business relationship with any company whose stock is mentioned in this article.
Biotechvalley Insights (BTVI) is not a registered investment advisor, and articles are not targeted toward retail investors. The content is for informational purposes only; you should not construe any such information or other material as legal, tax, investment, financial, or other advice. Nothing contained in our articles or comments constitutes a solicitation, recommendation, endorsement, or offer by Biotechvalley Insights or any third-party service provider to buy or sell any securities or other financial instruments in this or in any other jurisdiction in which such solicitation or offer would be unlawful under the securities laws of such jurisdiction. The research and reports made available by BTVI reflect and express the opinion of the applicable BTVI entity as of the time of the report only. Reports are based on generally-available information, field research, inferences, and deductions through the applicable due diligence and analytical process. BTVI may use resources from brokerage reports, corporate IR, and KOL/expert interviews that may have a conflict of interest with the company/assets that BTVI covers. To the best of the applicable BTVI's ability and belief, all information contained herein is accurate and reliable, is not material non-public information, and has been obtained from public sources that the applicable BTVI entity believes to be accurate and reliable. However, such information is presented “as is” without warranty of any kind, whether express or implied. With respect to their respective research reports, BTVI makes no representation, express or implied, as to the accuracy, timeliness, or completeness of any such information or with regard to the results to be obtained from its use. Further, any analysis/comment contains a very large measure of analysis and opinion. All expressions of opinion are subject to change without notice, and BTVI does not undertake to update or supplement any reports or any of the information, analysis, and opinion contained in them.
Seeking Alpha’s Disclosure: Past performance is no guarantee of future results. No recommendation or advice is being given as to whether any investment is suitable for a particular investor. Any views or opinions expressed above may not reflect those of Seeking Alpha as a whole. Seeking Alpha is not a licensed securities dealer, broker or US investment adviser or investment bank. Our analysts are third party authors that include both professional investors and individual investors who may not be licensed or certified by any institute or regulatory body.
