Summary:
- ZYNE has a cannabidiol gel it is running through various trials.
- The company and this drug have a history of failures.
- Despite some positives in a subgroup analysis, the failures do not inspire confidence.

Zynerba (NASDAQ:ZYNE) is a mid stage clinical company focusing on pharmaceutically-produced transdermal cannabinoid therapies for rare and near-rare neuropsychiatric disorders. Its pipeline is here:
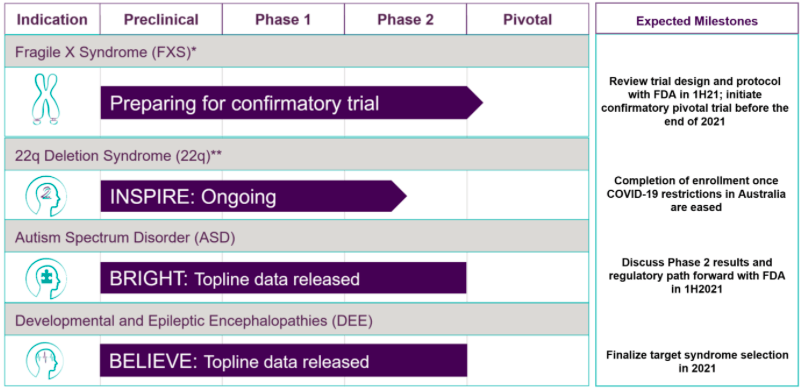
The company has only one product candidate being run through these various trials. This product is Zygel, a cannabidiol gel. Lead indication is Fragile X Syndrome or FXS, where it has completed phase 2 trials and will begin a confirmatory trial by 2021.
FXS is a rare genetic condition affecting approximately 71,000 Americans and with no approved drugs. It causes intellectual disability, anxiety disorders, social avoidance, behavioral and learning challenges and various physical characteristics. It is the leading known genetic cause of both inherited intellectual disability and autism spectrum disorder. Patients are born with a mutated gene responsible for regulating the endocannabinoid (EC) system. Zygel modulates multiple receptors and mediates numerous pathways, including the endocannabinoid pathway. It is the first and only patent- protected, permeation-enhanced, pharmaceutically-produced transdermal cannabidiol gel, with patent protection up to 2038.
Trial data
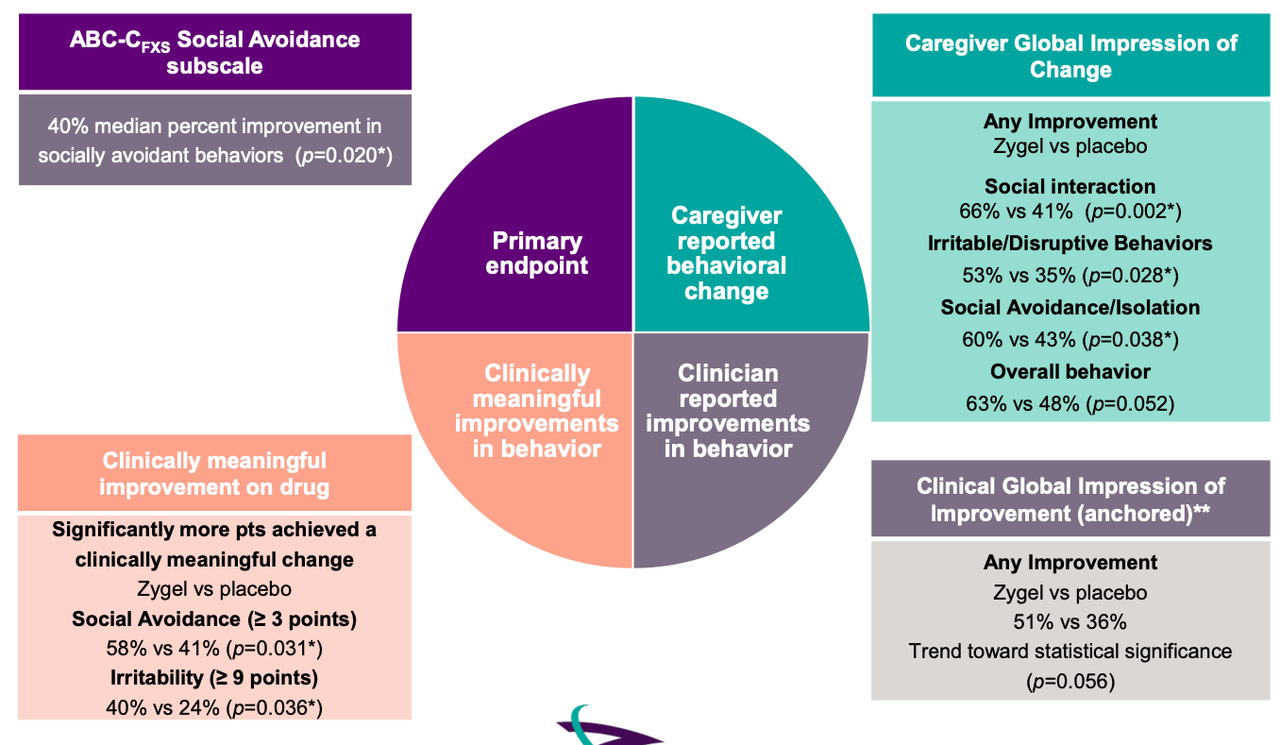
A phase 2 trial called CONNECT-FX has been run by the company comparing Zygel to placebo in 212 patients, both children and adolescents. A set of patients with ≥90% FMR1 methylation (167 total ~80% of trial population) have been included in pre-planned ad hoc analyses. The primary endpoint was: change from baseline to end of treatment in ABC-CFXS Social Avoidance subscale. Key secondary endpoints were: Change from baseline to end of the treatment in –
- ABC-CFXS Irritability subscale score
- ABC-CFXS Socially Unresponsive/Lethargic subscale score
- Improvement in Clinical Global Impression (CGI-I) at end of treatment, anchored to FXS behaviors
Efficacy data was as follows:
According to this data, the trial failed to meet its primary endpoint with statistical significance. However, in a subgroup of severely impacted patients defined as those having at least 90% methylation (full methylation) of the impacted FMR1 gene, Zygel met the primary endpoint. This group/comprised 80% of the total enrolled patient population. Full methylation occurs in ~60% of FXS patients.
The drug was well tolerated, with no serious adverse events. The most significant adverse event was application site pain. There were 7 patients with psychiatric disorder TEAEs; 5 were in the placebo group.
For approval, Zynerba plans to conduct a single double blind, placebo controlled pivotal trial in patients with a highly methylated FMR1 gene to confirm results seen in CONNECT- FX responders. The primary endpoint of this confirmatory trial will be the same as in the phase 2 trial. The difference will be that they will focus on the patient population where, in post hoc analysis, they saw statistically significant improvement.
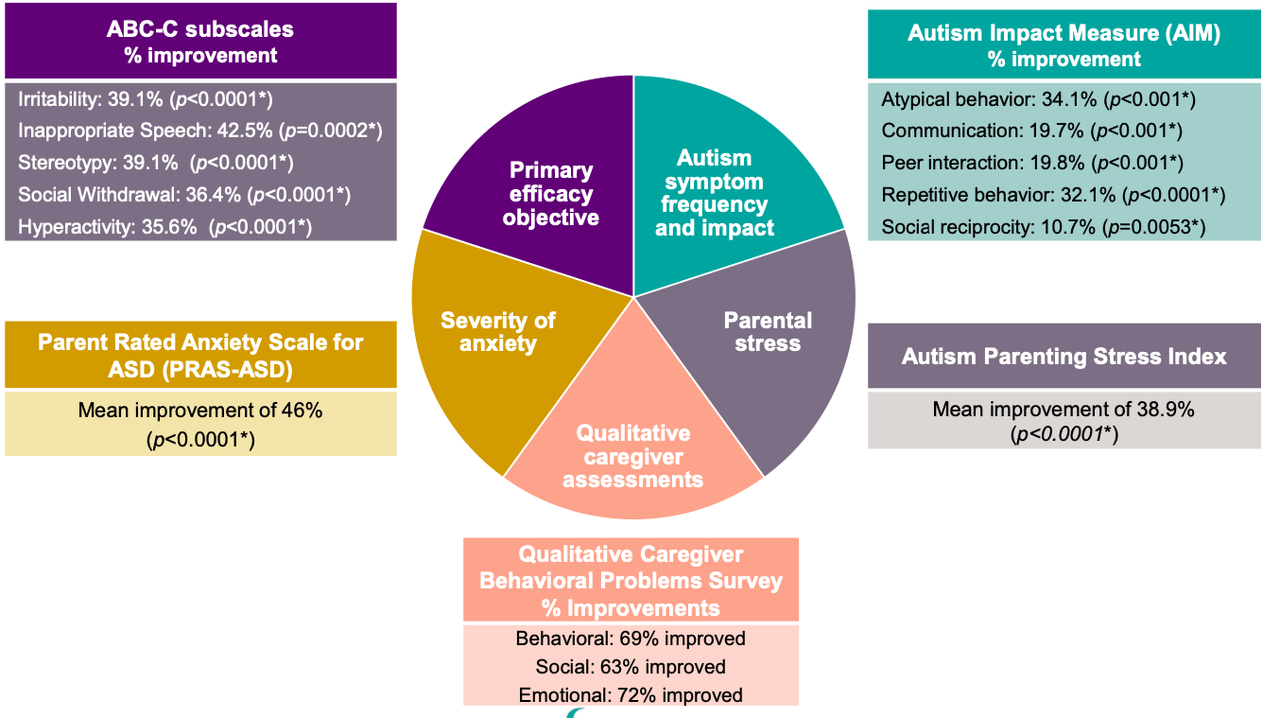
The company also released results from the BRIGHT phase 2 trial in autism spectrum disorder. Statistically significant results were seen at 14 weeks compared to baseline.
A trial in children with Developmental and Epileptic Encephalopathy was also successful in phase 2.
Financials
ZYNE has a market cap of $175mn and a cash balance of $59mn as on December 31, 2020. From January 1, 2021 to February 9, 2021 they raised net proceeds of $42.2 million through the sale of 10.2 million shares of equity through their “At The Market” sales agreement, which, adding to the previous balance, the company says is enough to support operations to 2024.
Bottom line
There was a time when Zynerba hoped to rival GW Pharma in the cannabinoid drug development. However, while GW Pharma was bought out by Jazz Pharma in a $7.2bn deal, Zynerba still remains a nanocap. This is because, in the last 4 years, it has failed four trials – epilepsy, osteoarthritis, a trial for THC as a patch, and this FXS trial. However, there may be some light at the end of the tunnel. Here, it could be in the subgroup analysis which will now form a basis for the confirmatory trial. However, the repeated failures do not inspire confidence in investors. We plan to remain on the sidelines.
Analyst’s Disclosure: I/we have no positions in any stocks mentioned, and no plans to initiate any positions within the next 72 hours. I wrote this article myself, and it expresses my own opinions. I am not receiving compensation for it (other than from Seeking Alpha). I have no business relationship with any company whose stock is mentioned in this article.
Seeking Alpha’s Disclosure: Past performance is no guarantee of future results. No recommendation or advice is being given as to whether any investment is suitable for a particular investor. Any views or opinions expressed above may not reflect those of Seeking Alpha as a whole. Seeking Alpha is not a licensed securities dealer, broker or US investment adviser or investment bank. Our analysts are third party authors that include both professional investors and individual investors who may not be licensed or certified by any institute or regulatory body.
About the TPT service
Thanks for reading. At the Total Pharma Tracker, we offer the following:-

Our Android app and website feature a set of tools for DIY investors, including a work-in-progress software where you can enter any ticker and get extensive curated research material.
For investors requiring hands-on support, our in-house experts go through our tools and find the best investible stocks, complete with buy/sell strategies and alerts.
Sign up now for our free trial, request access to our tools, and find out, at no cost to you, what we can do for you.