The Verdict On Aerie Pharmaceuticals
Summary:
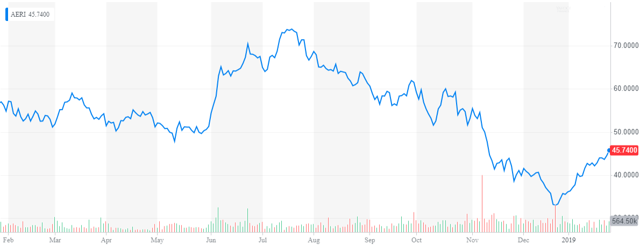
- We revisit Aerie Pharmaceuticals. The stock has had a wild ride, getting crushed in the back half of 2018 and rising nicely so far in 2019.
- The company is just rolling out its first approved product and has another PDUFA date with the FDA coming up in mid-March.
- We take a deeper look at this ocular concern in the paragraphs below.
“He has all the virtues I dislike and none of the vices I admire.” -Winston S. Churchill, Wealth, War, and Wisdom
It has been quite a while since we looked at Aerie Pharmaceuticals (NASDAQ:AERI). The stock, like most small biotech stocks, was crushed in the back half of 2018. However, the shares have acquitted themselves well so far in 2019. I have gotten a few questions from around this name recently. With a key PDUFA date in mid-March, it is time to revisit this ocular concern.
Company Overview:
Aerie Pharmaceuticals is a North Carolina based ophthalmic pharmaceutical company, focuses on the development and commercialization of first-in-class therapies for the treatment of glaucoma and other eye diseases. The stock came public late in 2013 and sports a market capitalization just north of $2 billion. It stock currently trades for just over $45 a share after a nice rally in January.
Product Portfolio & Pipeline:
Source: Company Website
Rhopressa was approved to treat glaucoma late in 2017 and rolled out in the first half of 2018. Rhopressa is a once daily treatment that lowers eye pressure in patients with open-angle glaucoma and ocular hypertension. In the third quarter, the compound did $7.2 million in revenues. The company expects Rhopressa will generate between $20 million to $30 million in sales in FY2018.
Aerie’s next potential approval is for Roclatan, which has a March 14th PDUFA date. Roclatan is a once-daily eye drop that combines netarsudil – the active ingredient in its approved product, Rhopressa® 0.02% – with latanoprost, prostaglandin analog (PGA) that is the most commonly prescribed medication for lowering Inter Ocular Pressure (IOP) to treat glaucoma.
In recent news, ten days ago the FDA accepted Aerie’s IND application for AR-1105 (dexamethasone intravitreal implant) for the treatment of macular edema due to retinal vein occlusion. A Phase 2 clinical study is expected to begin later this quarter.
Analyst Commentary & Balance Sheet:
The current median analyst price target on Aerie is just north of $80 a share. So far in 2019, both Cantor Fitzgerald ($86 price target) and Mizuho Securities ($77 price target) have reissued Buy ratings on the stock. Here is the commentary from Cantor’s call which came out on January 9th:
We rate Aerie Pharmaceuticals Overweight. AERI is commercializing and developing once-daily eye drops, respectively, for glaucoma. Rhopressa is the first novel drug in a market that has not seen innovation in 20 years. Valuation Summary We value Aerie based on the NPV of the cash flows associated with its glaucoma drug candidates. Our target price is $86/share.
The company came out of the third quarter with just over $235 million of cash on hand. Management guided that it expected cash burn for FY2018 to be in the $210 million to $215 million range after burning through $163 million in the first nine months of 2018.
Verdict:
Aerie looks poised to add a second compound to its product portfolio in mid-March and continues to advance its pipeline. The stock also enjoys solid analyst support and could continue to run up a bit into its March PDUFA date.
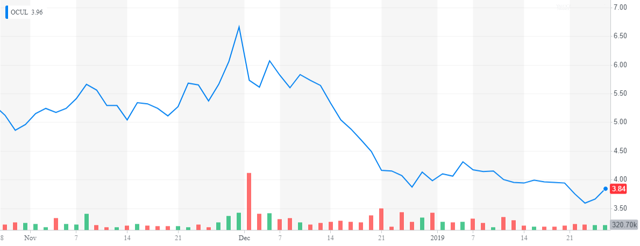
That said, we have seen sell-offs after FDA approvals in the biotech space overall including in fellow ocular concern Ocular Therapeutix (OCUL) whose product DEXTENZA received FDA approval on December 3rd of last year. In addition, it appears the company is going to have to raise additional funding sometime this year. Finally, insiders were frequent sellers of the shares throughout 2018.
I have a small “watch item” holding in AERI. I have no plans to add to my holdings currently and if I was going to take an initial position in AERI, it would be through a buy-write option order. Post approval/capital raise, I may revisit this name to see if the risk/reward profile has improved. I offer this analysis up for the several folks that have inquired on this name recently.
“No one gossips about other people’s secret virtues.” – Bertrand Russell
Bret Jensen is the Founder and author of articles on The Biotech Forum, The Busted IPO Forum, and The Insiders Forum. To receive these articles as published on Seeking Alpha, just click the appropriate link and hit the orange follow button.
Disclosure: I am/we are long AERI, OCUL. I wrote this article myself, and it expresses my own opinions. I am not receiving compensation for it (other than from Seeking Alpha). I have no business relationship with any company whose stock is mentioned in this article.
Biotech has gotten off to a solid start 2019 after deep losses in the fourth quarter. M&A has picked up as two major acquisitions have happened early this year. We just provided our 2019 Outlook to Biotech Forum members. It contains what we expect in this high beta sector for the rest of year as well as a couple of possible logical takeout candidates. To get it, access to our model portfolio and our investment analysis archives, sign up for your free 14 day trial into The Biotech Forum by clicking here. Hope to see you on Live Chat soon!